Sourced by Dr Vanessa Panettieri, Educational Editor
Welcome back to the featured papers in the AFOMP journals! Getting close to our 25th birthday we reflect on some advanced technologies and specialised treatments and look at the importance of understanding and mastering the art of dosimetry. In the first contribution we explore the use of different detectors for small field dosimetry. A crucial step when moving from more conventional treatment to ultra-hypofractionation. We further explore radiosurgery applications and compare different modalities for the treatment of trigeminal neuralgia, trying to answer the question: if you could have any technology what would you choose for trigeminal neuralgia treatment? We then move to dose enhancement and look at the effect of using nanoparticles in breast treatments. Finally we conclude our featured papers section with two comprehensive review articles exploring AI applications in diagnostic imaging medical physics and for brain tumour segmentation.
Happy reading and let the Editorial team know if there is any topics you would like us to explore in detail for the next issue!
With contributions kindly provided by Sadia Aftab, Medical Physicists, Peter MacCallum Cancer Centre
Focus on: small field dosimetry

https://doi.org/10.1007/s13246-025-01546-w
This first study published by Habib et al, published in the Physical and Engineering science in Medicine Journal, investigates the challenges of accurately measuring and modelling radiation dose in small fields which are commonly used in modern radiotherapy techniques such as IMRT, VMAT, and stereotactic treatments. The authors explore different methodologies to address the challenge of small field dosimetry including both measurements with detectors and Monte Carlo simulations. The aims of the study are to analyse how variations in full-width-half-maximum (FWHM) of the Gaussian distribution of the primary electron source affect radiotherapy doses for small fields. The authors have conducted comparisons between dose calculations performed using an ideal definition of the size of the electron source, and then by using a modified definition that relies on an asymmetrical shape of the source. To understand the effect of each model the authors have performed measurements using a range of detectors including the Razor diode and then have also used four different planning cases (nasopharynx, astrocytoma, cerebellum and breast) to evaluate the impact of the minimum segment width in treatment planning delivery and accuracy. The authors have planned each case with a ranging minimum segment width from 0.5 to 1 cm and then measured each case using a 2D-array and assessing gamma pass rates. The work concludes that inaccurate modelling of the primary source FWHM can cause significant errors in dose calculation when using a MC model of the beam, which in turn can result into an inaccurate delivery of the patient treatment. These inaccuracies become unacceptable for fields below ≤0.5×0.5 cm². As well described in dosimetry protocols detector choice when measuring these fields is crucial. Finally when planning the treatment in the TPS limitations should be considered when shaping the beam by adding limitations on the beamlet width. The authors suggest that recommends that in IMRT/VMAT planning a minimum beamlet/segment size ≥1 cm² should be recommended in addition to robust QA processes for safe and effective treatment. Keen to discuss more this topic in the next issues!
Focus on advanced applications:
- Radiosurgery for trigeminal neuralgia

https://doi.org/10.1007/s12194-025-00935-w
This study by Kannan M. et al., published in Radiological Physics and Technology, presents a comprehensive dosimetric evaluation of three stereotactic radiosurgery (SRS) modalities – Gamma Knife (GK), CyberKnife (CK), and Linear Accelerator (LA) for the treatment of trigeminal neuralgia (TN), a chronic condition marked by severe facial pain. While GK has traditionally been the gold standard for SRS in TN, the emergence of CK and LA-based techniques has prompted interest in comparing their dosimetric performance and treatment efficiency. The primary aim of the study was to assess whether CK and LA could match GK in terms of target dose coverage, conformity, critical structure sparing (particularly the brainstem), low dose spread to surrounding tissues, and overall planning and delivery efficiency.
The findings revealed that CK and LA achieved comparable target coverage to GK when delivering a 60 Gy dose. However, GK demonstrated superior brainstem sparing and significantly lower low-dose exposure to normal tissues, attributed to its high beam count (192 beams). Statistically significant differences were observed in several dose metrics—such as (e.g., DMin,D98,D90,D50,D30 ) between GK and the other two modalities, but not between CK and LA. GK also delivered significantly lower doses to the brainstem in parameters like D0.03cc,D1,D2 and had the lowest volume of tissue receiving 4 Gy. No significant differences were found in doses to the optic nerves, cranial nerves VII/VIII, or eyeballs, though temporal lobe doses were lower with GK.
The study concludes that while GK remains the preferred modality due to its dosimetric advantages, CK and LA are viable alternatives, particularly in settings where GK is unavailable. The authors highlight the need for further research into clinical outcomes, such as pain relief, recurrence rates, and side effects—to better inform treatment decisions across these modalities.
- Effect of gold nanoparticles in RT of breast cancer
https://doi.org/10.1007/s12194-025-00919-w
This study by Murat Aygün and Zeynep Aygün, published in Radiological Physics and Technology, investigates the impact of gold nanoparticles (AuNPs) on the effectiveness of radiation therapy for breast tumours. The research explores how varying concentrations of AuNPs influence radiation interactions with breast tissue under different irradiation types, including photons, electrons, protons, helium ions, carbon ions, and neutrons. Using advanced simulation tools such as PHITS, SRIM, ESTAR, PAGEX, and Phy-X/PSD, the authors calculated key radiation interaction parameters like mass attenuation coefficients, stopping power, radiation yield, and particle range.
The results demonstrate that the presence of AuNPs significantly enhances radiation absorption in tumour tissues. Higher concentrations of AuNPs lead to increased attenuation coefficients and energy deposition, particularly evident in photon and neutron interactions. For charged particles, the inclusion of AuNPs shifts the Bragg peak closer to the tumour surface, intensifying dose delivery at the target site while reducing penetration depth. This effect was most pronounced with carbon ions, followed by helium and protons. Additionally, the study found that AuNPs improve neutron absorption beyond that of lead, a commonly used shielding material.
Overall, the findings suggest that AuNPs can substantially improve the precision and efficacy of radiation therapy by increasing dose deposition in malignant tissues while potentially sparing healthy ones. The study concludes that incorporating AuNPs into tumour regions could reduce treatment time and enhance therapeutic outcomes. However, the research is theoretical and simulation-based, and further experimental and clinical validation is necessary to confirm these benefits and assess safety and biological compatibility.
Focus on: AI applications for diagnosis and tumour segmentation

https://doi.org/10.1007/s13246-025-01535-z
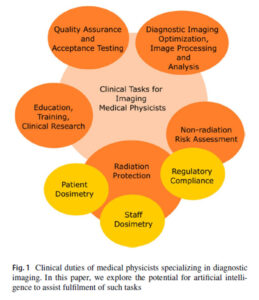
In this first review by Lam and Ng the authors reflect on the role of AI in the clinical practice of Diagnostic Imaging Medical Physicists, who hold vital responsibilities across healthcare, ensuring that imaging equipment operates safely and effectively, while also managing radiation dosimetry, protection, and risk assessment. Their expertise extends into image analysis, research, education, and compliance with legal and regulatory frameworks. As imaging technologies become increasingly sophisticated, with innovations such as dual-energy CT, solid-state gamma detectors, and AI-driven reconstruction, the demands on physicists continue to grow, with an increase need for an upskilled workforce. Artificial intelligence is emerging as a powerful ally in radiology and medical physics, with applications ranging from image acquisition and reconstruction to analysis tasks like detection, classification, and segmentation. Beyond improving image quality and efficiency, AI also has the potential to streamline workflow by automating quality assurance checks, trend monitoring, and report generation. In radiation safety, it could enable more precise dosimetry and faster risk assessments, while also supporting education and training through data-driven simulations and decision-support tools (Fig 1).
Despite these promising opportunities, clinical adoption of AI in medical physics remains limited, with significant challenges ahead. Validation, reliability, and seamless integration into existing workflows are essential, as is building trust through training and transparency. Regulatory and legal frameworks must also evolve to ensure safe implementation. The outlook, however, is optimistic: with focused research, pilot studies, and collaborative efforts between physicists, AI developers, and clinical teams, AI could become a transformative force, enhancing efficiency, safety, and innovation in imaging physics.

DOI: 10.4103/jmp.jmp_12_25
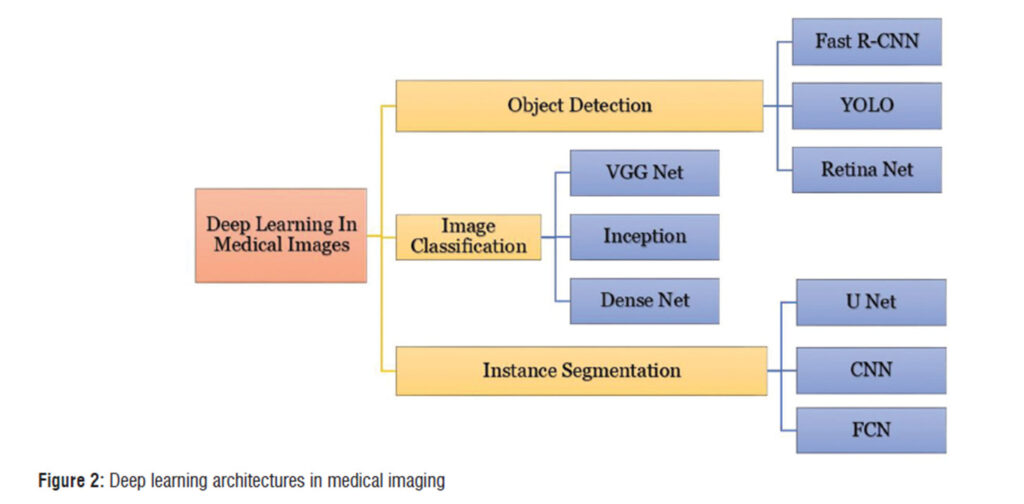
This review by Purohit and Bhatt provides a comprehensive overview on how brain tumour segmentation has advanced from manual methods to deep learning techniques. This is of clinical interest due to the critical importance of brain tumour segmentation in all the steps of the treatment workflow from diagnosis, to treatment planning, delivery and follow-up. This review examines various deep learning (DL) architectures used for segmentation tasks, and highlights how Convolutional neural networks (CNNs) and newer architectures like U-Net, V-Net, and transformers are setting new standards in accuracy and efficiency (Fig 2 in the paper provides a diagram of the architectures used in medical Imaging).This is in addition to optimizers such as Adam, SGD, and newer variants like Ranger21 which play a critical role in fine-tuning these models, directly impacting their precision and speed.
The authors demonstrate that deep learning approaches have achieved remarkable performance, with Dice scores as high as 0.91 and validation accuracies up to 98%, clearly outperforming traditional methods. They also provide a clear summary of the advantages and limitations of all the algorithms used for these applications, and of the optimisers’ significance in Table 1 and 2 of the paper making them a good reference for researchers looking in this field.
AI-driven methods are advancing tumour detection, treatment planning, and patient monitoring by integrating multimodal imaging and advanced MRI techniques, which further improve segmentation accuracy. However, key challenges remain, including high computational demands, dataset imbalance, and limited generalization across diverse clinical settings. Looking ahead, the authors emphasize the importance of transfer learning, explainable AI, and larger multi-institutional datasets to build models that are not only more accurate but also robust, interpretable, and suitable for routine clinical practice.
