Sourced by Assoc.Prof. Vanessa Panettieri
Modeling the Effect of Daughter Migration on Dosimetry Estimates for [225Ac]Ac-DOTATATE
Stephen Tronchin, Jake Forster, Kevin Hickson, Eva Bezak, International Journal of Radiation Oncology*Biology*Physics, Volume 122, Issue 5, 2025, Pages 1356-1368, ISSN 0360-3016.
https://doi.org/10.1016/j.ijrobp.2025.03.004
What inspired this study?
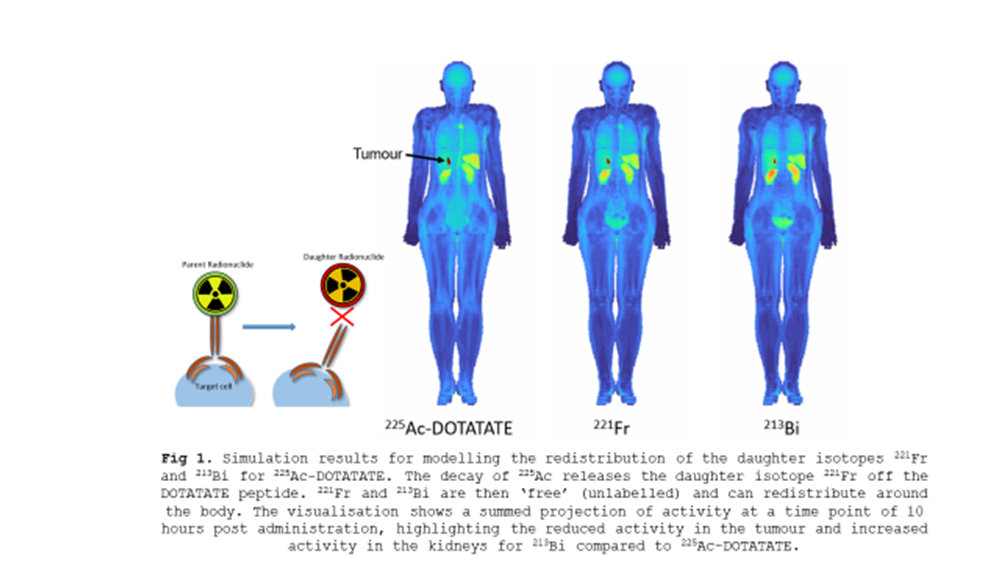
This study was aimed at addressing a key concern in targeted alpha therapy (TAT) with actinium-225 (225Ac) – namely, the effect of daughter radionuclide migration. The high recoil energy produced during the alpha decay of 225Ac can break the bond to the targeting peptide (DOTATATE), resulting in free daughter radionuclides being released in the body. Since daughter migration is generally not accounted for in standard clinical dosimetry, this could lead to inaccurate dosimetry estimates. We developed a model to simulate the unique biokinetics of the daughter isotopes, and quantified how daughter radionuclide migration affects organ and tumour absorbed dose estimates.
What were the key challenges in this research?Most pre-existing compartment models are designed for parent radionuclides, and not for dynamically relocating daughters. Therefore, we had to develop a custom compartment model to simulate the biokinetics of the full 225Ac decay chain. Furthermore, the time-activity curves of 225Ac-DOTATATE are required as input into the model. In TAT, a surrogate radionuclide suitable for imaging is generally used to obtain biodistribution information on the targeting vehicle. We utilised published imaging data of 111In-DOTATATE to estimate the biodistribution of 225Ac-DOTATATE, which comes with limitations.
What are the key takeaways from this study?
Standard clinical dosimetry models tend to assume all the daughter isotopes remain at the decay site of the parent, which may produce inaccurate dose estimates. From the 11 patients we simulated, we found that the effect of daughter migration increases the kidney dose estimate by approximately 10%, while decreasing the tumour dose estimate by approximately 23%. Therefore, ignoring the effect of daughter migration can underestimate the absorbed dose to the kidneys and overestimate the absorbed dose to the tumours. Therefore, the therapeutic index may be overestimated when daughter migration is ignored.
How does this research impact the future?
By demonstrating the kidney may receive a higher dose than expected due to daughter radionuclide migration, this could help inform risk assessments for patients undergoing therapy with 225Ac-DOTATATE. The model could also be used for patient specific dosimetry in a clinical setting, provided time-activity curves of an imaging radionuclide are obtained. This can help ensure the tumours are receiving a therapeutic dose, while ensuring the normal organs remain below threshold levels. This work may also help determine the optimal activity to administer to patients, particularly during clinical trial phases. By improving dosimetry estimates, it is possible to adjust the administered activity to ensure the tumours and normal organs are receiving the optimal dose. We hope this work will help optimise TAT therapies and provide valuable information for clinical trials with 225Ac-labelled radiopharmaceuticals.