Targeted radionuclide therapy in nuclear medicine: PSMA Theranostics and beyond
Lachlan McIntosh, PhD student, Peter MacCallum Cancer Centre, Australia
As a student of nuclear medicine, one of the most amazing aspects of the field to me is its multidisciplinary nature and how it commands the collaboration of many scientific and medical disciplines. Advancements in the field of nuclear medicine, specifically in targeted radionuclide therapy, requires specialised physics input where we, as the medical physicists, can assist in optimising and personalising treatments, as well as providing clinical staff with relevant safety guidance to confidently carry out the procedures.
Since 2014, there has been a rapid increase in the implementation of PSMA-bound (prostate-specific membrane antigen) radiopharmaceuticals for diagnostics and therapy in the treatment of prostate cancer (PCa). Once identified, local PCa has several immediate treatment options, such as surgery (radical prostatectomy), external beam radiotherapy (EBRT), brachytherapy, and active surveillance for low-risk cases. Once spread to oligometastatic form, treatment options focus on EBRT to metastatic sites and systemic therapies like androgen deprivation therapy (ADT). Once in the widely spread metastatic form, previous treatments become less effective and almost futile with remaining options being chemotherapy agents (cabazitaxel), hormonal or immunotherapy agents (abiraterone, enzalutamide), and more recently, PSMA radionuclidetherapy. The first proof-of-concept human studies were completedover a decade ago, with the first publication describing the theranostic potential of combining similar targeting agents for both diagnostic and therapeutic approaches. In this manner, the PSMA targeting molecule is the key driver of isotope clearance and retention where the diagnostic Ga-68 PET image is used to guide treatment decisions and overall management.
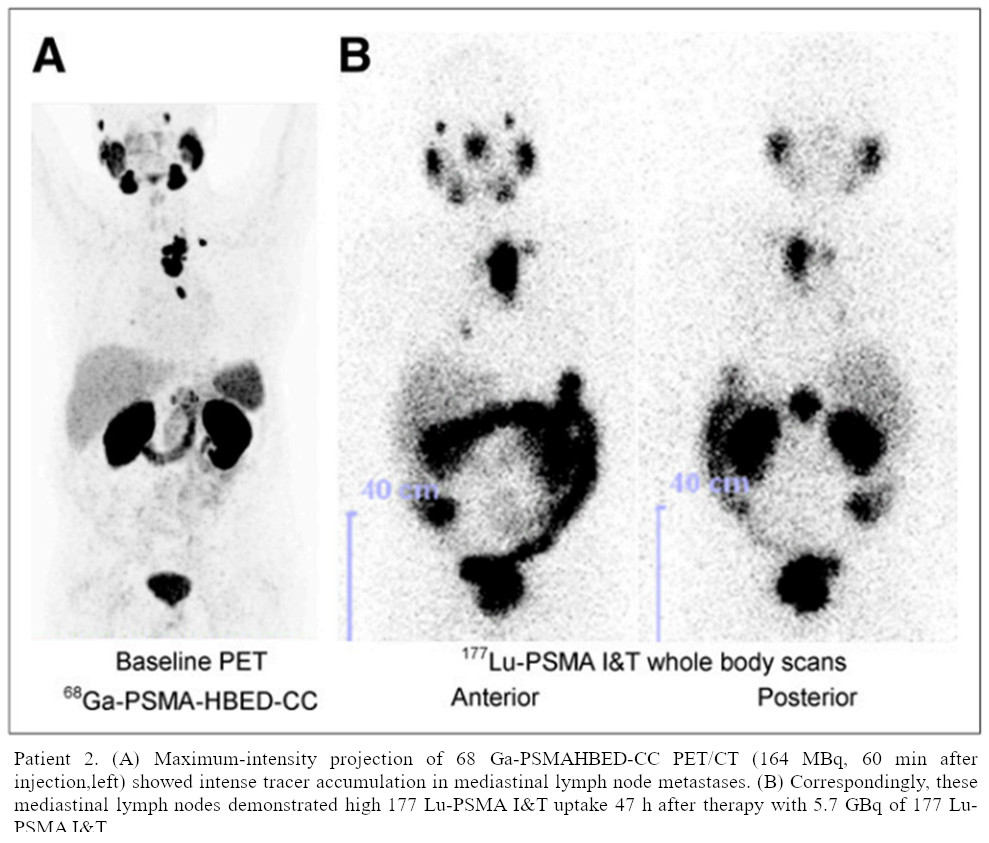
Targeted radionuclide therapy (TRT) is ideal for managing metastatic cancers that have spread throughout the body, where local treatments like surgery or external beam radiation are ineffective. TRT can even target and treat microscopic cancer cells that are undetectable by current imaging methods, reducing the risk of recurrence and progression. The high affinity of targeting molecules for cancer-specific antigens minimises radiation exposure to normal tissues and allows higher localised radiation doses to be delivered to cancer cells while sparing healthy organs and minimising side effects depending on molecular specific pharmacokinetics. As each targeting molecule is designed to bind selectively to antigens or receptors that are overexpressed on a specific cancer cell or even multiple types of cancer cells, such as the novel work with fibroblast-activation-protein inhibitors FAPI PET [1], the diagnostic imaging patterns can be utilised for estimations of the higher dose therapeutic treatment delivery.
Isotopes for imaging compared to therapy
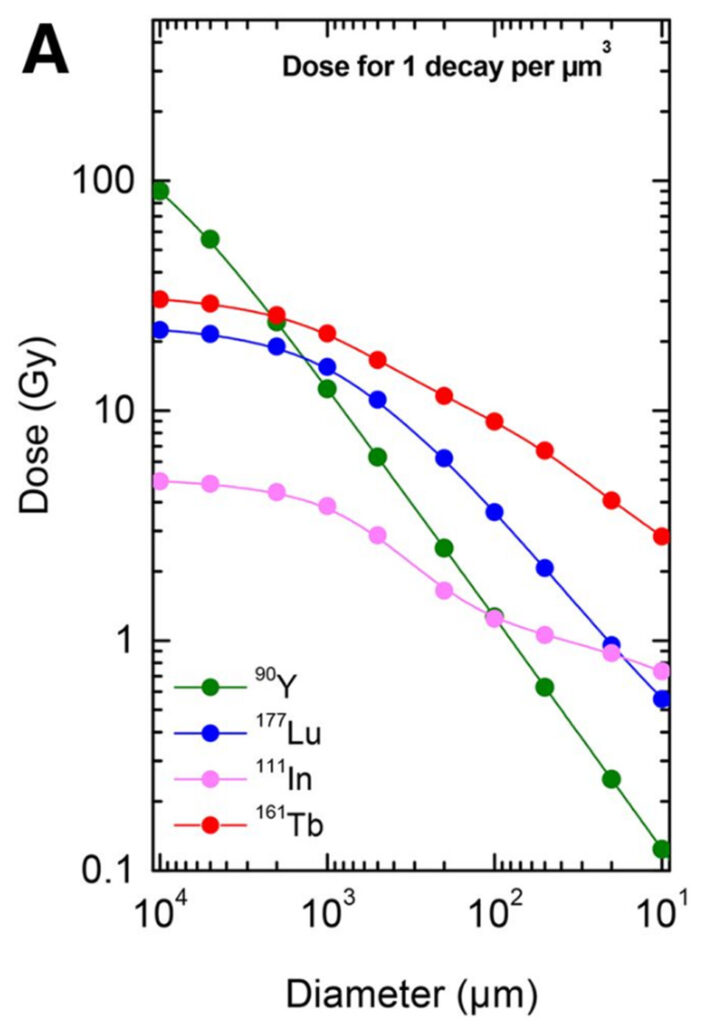
Solely of the purpose for imaging, positron emitters are used due to the ability for the 511 gamma pairs to penetrate tissues and provide clear diagnostic images with high reproducibility and resolution (F-18, Ga-68). Uptake patterns on Ga-68/F-18 PSMA PET and FDG PET scans help tailor Lu-177 PSMA therapy, optimising the therapeutic dose and minimising toxicity. I should note that this approach is not limited to PSMA although it has generated significant interest in this new era of targeted systemic therapies owing to the clinical successes of the many Lu-177-PSMA trials. The radioactive isotope employed in a radionuclide therapy treatment aims to emit radiation damage to the DNA of cancer cells at a known rate relative to the inherent decay properties, leading to localised cell death. These decay properties, separated distinctly by their initial mode of either alpha, beta, gamma, electron capture, or a combined sequence, define the energy deposition per nuclear transition for a given medium. The dose per nuclear transition in water for Y-90, Lu-177, In-111, and Tb-161 are featured in figure 2. In the case of beta therapy with Lu-177 and Tb-161 PSMA TRT, the decay products are stable where no other daughters exist. Conversely, in the case of alpha PSMA treatment with Pb-212 or Ac-225, there are several daughter products also existing in equilibrium which lead to a barrage of different emissions.Alpha treatments are given at a much lower activity level (~7000 MBq for a beta therapy with Lu-177, ~70 MBq for an alpha therapy with Pb-212) in accordance with their LET. The initial alpha decay consists of high-energy particles that travel an extremely short range, causing intense damage to nearby cells on the order of nm to mm. In contrast, beta radiation consists of lower-energy particles with a longer range, on the order of mm to cm, making it suitable for treating larger or more diffuse tumours. With most nuclear transitions of high energy, there is often cascade or de-excitation events that result in either emission or creation of gamma rays.While providing no tangible benefit to the therapeutic procedure in Lu-177, Tb-161, and Pb-212, they enable post-therapy SPECT/CT imaging — a key component of estimation in post-therapy PSMA dosimetry.
With a well calibrated SPECT imaging device, it is possible to infer total decays per unit voxel based on acquired counts at that specific energy window.
Post-therapy Dosimetry
Post-therapy dosimetry assesses the radiation dose delivered to tumours and normal tissues, guiding dose adjustments in subsequent treatment cycles. Dose reduction or enhancement is based on estimated organ at risk toxicity thresholds, typically of the kidneys of salivary glands for PSMA, to balance treatment efficacy and potential toxicity. To estimate delayed pharmacokinetics, serial timepoint imaging is used to calculate voxel-wise estimates of radiopharmaceutical clearance (Figure 3), or cohort population averages in the case of single-timepoint imaging used at our centre [4]. By implementing a two or three phase exponential relative to the number of serial images available, one can estimate the total nuclear transitions at a voxel level based on a few assumptions in terms of initial uptake and final washout based on spatially fused SPECT/CT imaging.
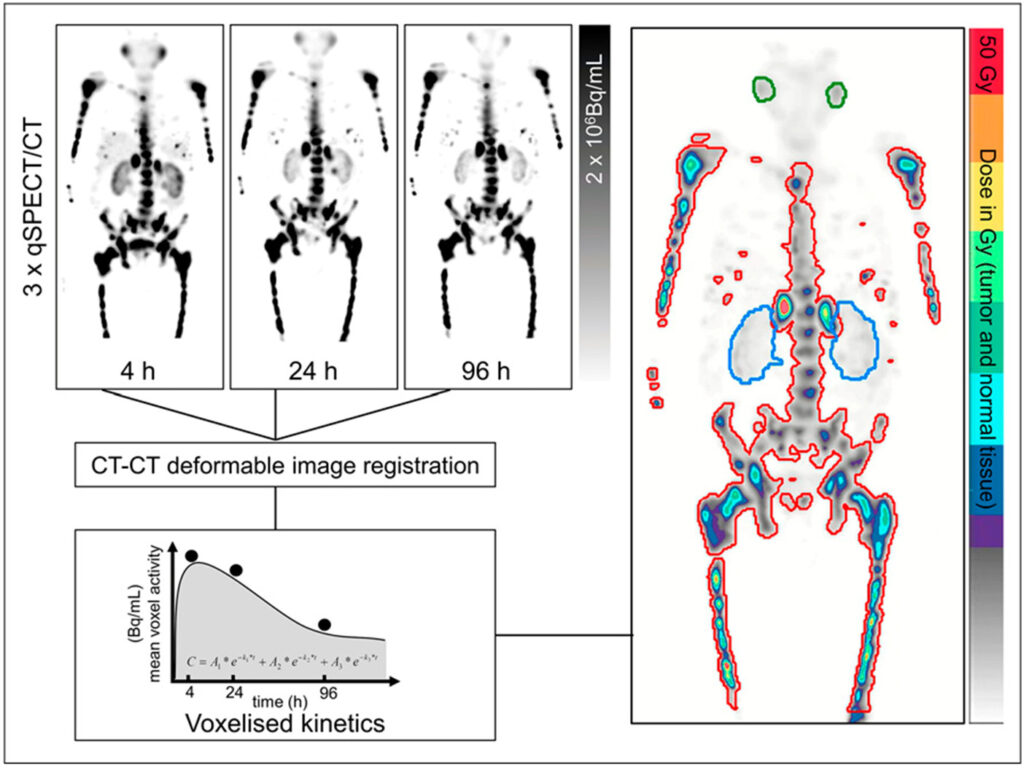
In conclusion, targeted radionuclide therapy leverages the specificity of targeting molecules and the cytotoxic effects of radioactive isotopes to provide an effective and personalised treatment option for cancer patients. The integration of diagnostic and therapeutic components, as well as deliberate selection of isotope relative to treatment aims, enhances the precision and efficacy of this treatment modality. The ongoing collaboration between the many disciplines involved is providing new treatment options for people that in the past may have exhausted all lines of therapy. I am hopeful to read future works regarding the advancement in targeting efficacy, further understanding of sub-cellular localisation of each molecule, and optimisation of therapeutic isotope choice to improve patient outcomes and durability.
References
- Kratochwil, C., Flechsig, P., Lindner, T., Abderrahim, L., Altmann, A., Mier, W., … &Giesel, F. L. (2019). 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. Journal of Nuclear Medicine, 60(6), 801-805.
- Weineisen, M., Schottelius, M., Simecek, J., Baum, R. P., Yildiz, A., Beykan, S., … & Wester, H. J. (2015). 68Ga-and 177Lu-labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. Journal of Nuclear Medicine, 56(8), 1169-1176.
- Hindié, E., Zanotti-Fregonara, P., Quinto, M. A., Morgat, C., & Champion, C. (2016). Dose deposits from 90Y, 177Lu, 111In, and 161Tb in micrometastases of various sizes: implications for radiopharmaceutical therapy. Journal of Nuclear Medicine, 57(5), 759-764.
- Jackson, P. A., Hofman, M. S., Hicks, R. J., Scalzo, M., & Violet, J. (2020). Radiation dosimetry in 177Lu-PSMA-617 therapy using a single posttreatment SPECT/CT scan: a novel methodology to generate time-and tissue-specific dose factors. Journal of Nuclear Medicine, 61(7), 1030-1036.
- Violet, John, Price Jackson, Justin Ferdinandus, Shahneen Sandhu, Tim Akhurst, Amir Iravani, Grace Kong et al. “Dosimetry of 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: correlations between pretherapeutic imaging and whole-body tumor dosimetry with treatment outcomes.” Journal of Nuclear Medicine 60, no. 4 (2019): 517-523.
